Renuva Scientific Timeline
Objective | Bench top tests to make sure cells will respond to Renuva and function.
Methodology | Adipose-Derived Stem Cells were layered on top of the matrix to simulate what happens in the body.
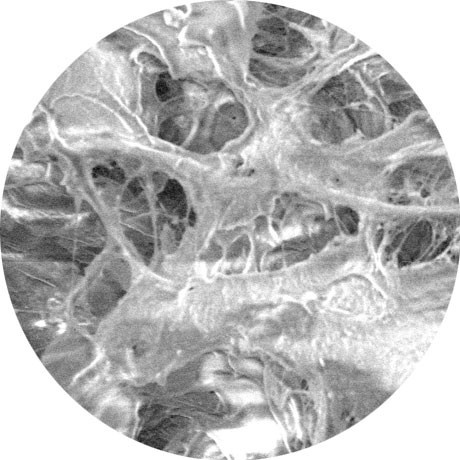
0 Days
Scanning Electron Microscope (SEM) picture of Renuva honeycomb structure (no cells added)
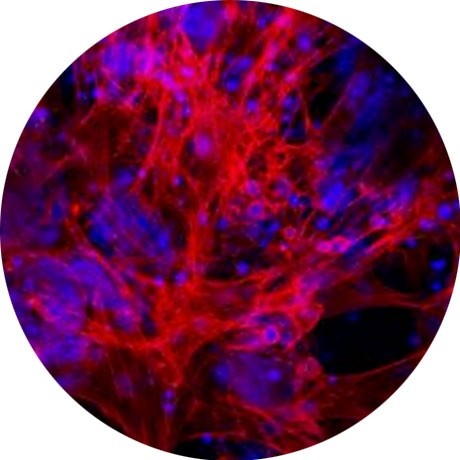
< 7 days
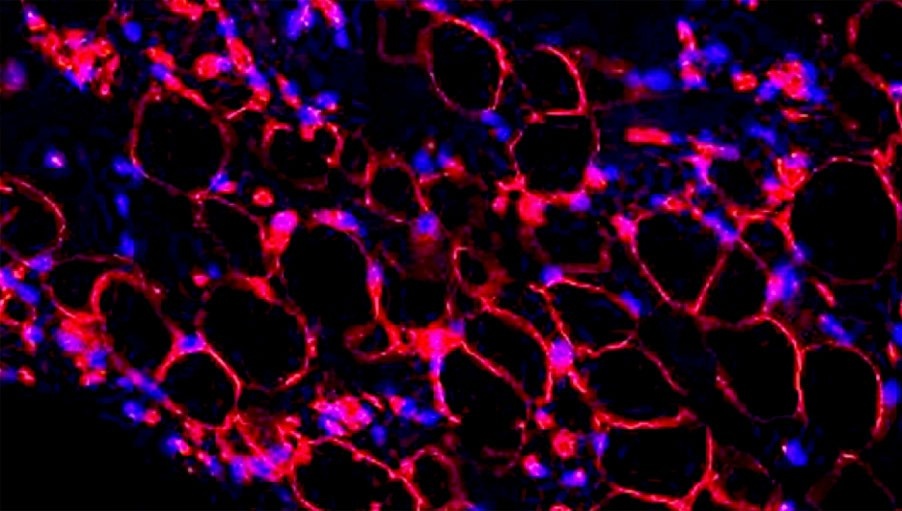
Red staining of the cell cytoskeleton indicates cell attachment and infiltration in the adipose scaffold
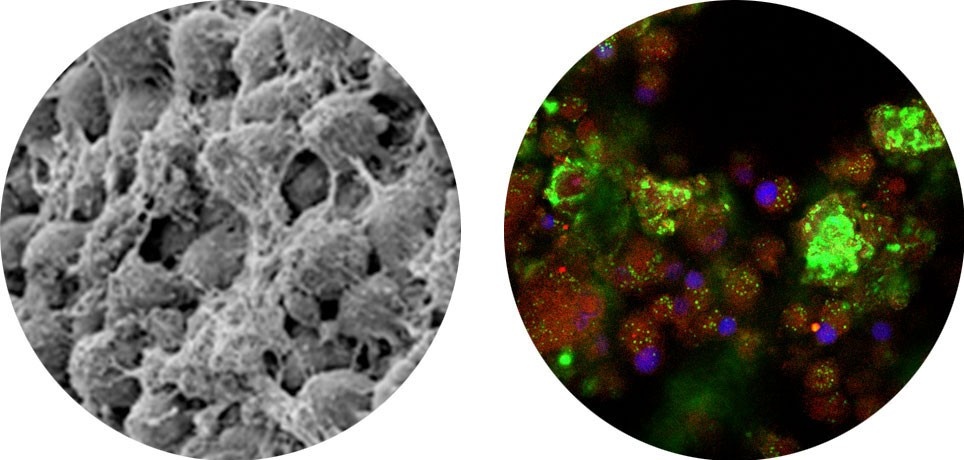
7 - 14 Days
ADSCs differentiate into adipocytes, secrete matrix proteins (SEM) (left) and accumulate intracellular lipid (green fluorescence = Bodipy, stains for lipid) (Blue fluorescence = DAPI – strain for nuclei) (right)
Result | Confirmation that the matrix supports the repopulation of adipocytes.
Objective | In vivo verification that Renuva supports blood vessel formation and adipogenesis.
Methodology | Renuva was injected subcutaneously in athymic mice and evaluated histologically at 24 weeks.
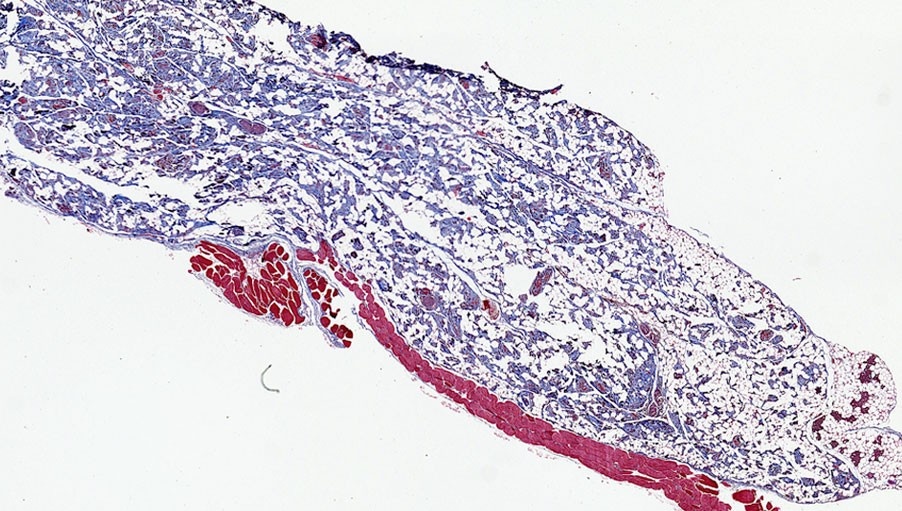
24 Weeks
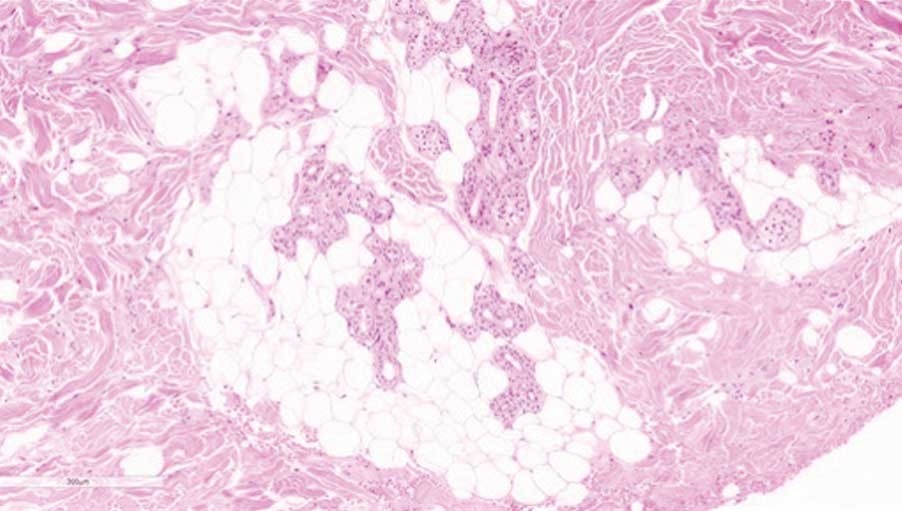
presence of adipocytes in the injected material
24 Weeks
adipogenesis confirmed with perilipin A
Result | Angiogenesis was observed initially. Adipogenesis was evident for up to 24 weeks.
Objective | Assess Renuva safety, short term volume retention, local skin changes, subcutaneous fat changes.
Methodology | Dr. Sydney Coleman and Dr. Roger Khouri perform clinical, and local injection site assessments. Study conducted over 16 weeks.
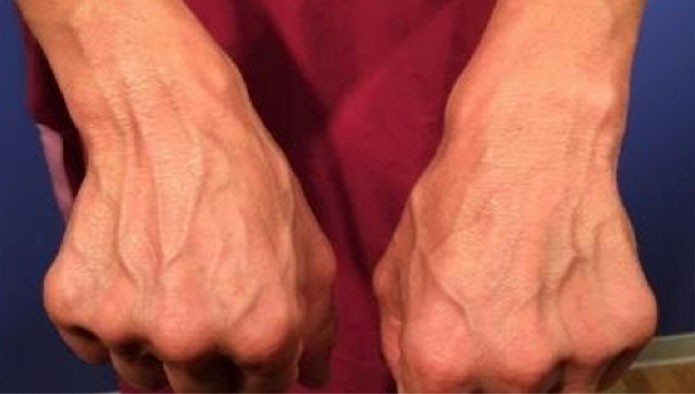
Single injection in dorsal wrist (2.5-5.5cc).
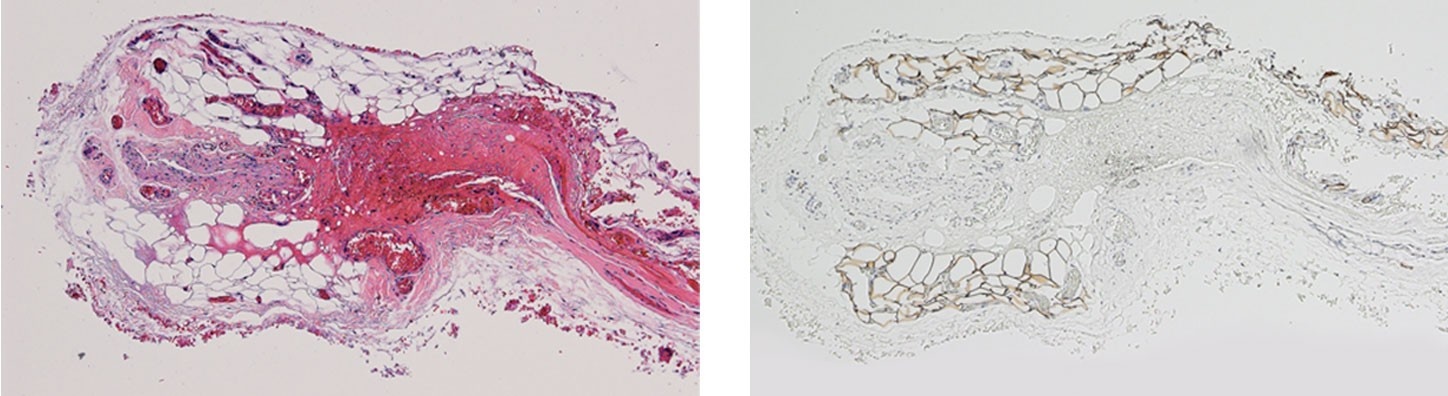
Hematoxylin and eosin (H&E - left) and Perilipin A (right) staining of a tissue biopsy at 16 months confirmed the presence of clusters of adipocytes where Renuva had been injected.
Result | No significant adverse events. All patients presented apparent thickening and enhancement of quality of the skin through completion. The prominence of veins and tendons diminished after injection and up to a year for returning patients.
Objective | Evaluate the remodeling of Renuva injected into the subcutaneous tissues.
Methodology | 6 injection sites per patient (20cc each).

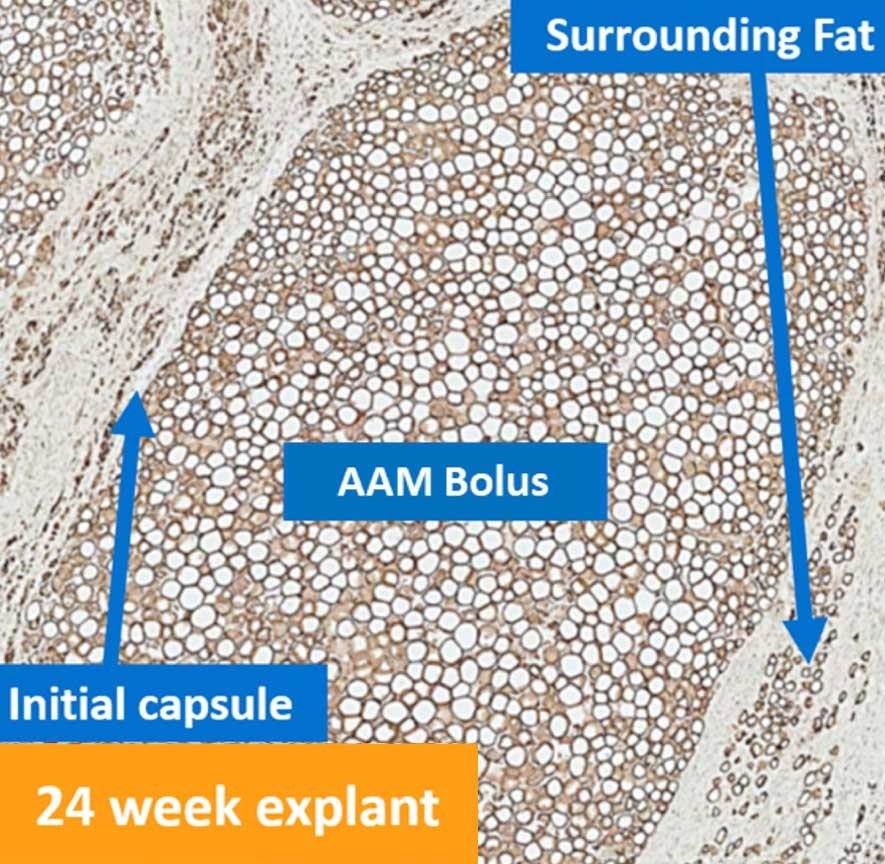
Result | Renuva can be injected as a 20cc bolus and still be populated - unlike fat. Renuva is easy to use, off-the-shelf, safe, and remodels into adipose tissue.
Objective | Evaluate clinical safety and retention of Renuva following injection in patients with bilateral atrophic temples.
Methodology | Up to 3cc injected per temple. Dr. Gold, Dr. Kinney, Dr. Kaminer and Dr. Rohrich evaluted results over 24 weeks.
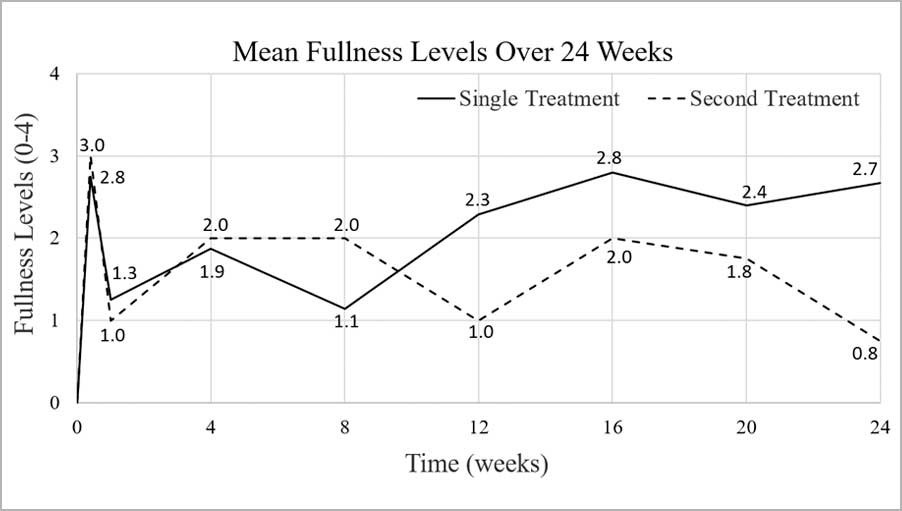
Result | Renuva is safe and well-tolerated in the face. Adipogenesis and angiogenesis was observed, along with improved skin quality. Retention at least 6 months.
Objective | Evaluate skin quality before and after Renuva injection. Evaluate longevity of result for up to 24 weeks. Evaluate safety for up to 24 weeks.
Methodology | Up to 2cc (malar), 2cc (pre-jowl), re-injection option at 12 weeks up to 1cc each. Half of the patients Half of the patients were treated with a regular concentration of Renuva and half with a diluted concentration of Renuva. 6 month follow-up.


4 prospective clinical studies as of today
- Renuva has been demonstrated to be safe in all studies
- Angiogenesis and adipogenesis have been observed
- Volume correction retained up to 6 months
- Continuing investment and new studies being designed and initiated.
Retrospective studies for craniofacial applications, continuing investment


